Abstract
Background SEA-BCMA is an investigational, humanized, effector enhanced, nonfucosylated IgG1 monoclonal antibody directed to BCMA. SGNBCMA-001 (NCT03582033) is an ongoing phase 1, open-label, multicenter, dose-escalation/expansion study evaluating the safety, tolerability, and antitumor activity of SEA-BCMA in adults with relapsed/refractory multiple myeloma (R/R MM). Part A comprised monotherapy dose escalation (100-1600 mg flat dosing by intravenous infusion) and dose expansion at the highest tolerated dose; SEA-BCMA 1600 mg once every 2 weeks (Q2W) was generally well-tolerated and showed initial antitumor activity with an overall response rate (ORR) of 14% (95% confidence interval [CI]: 2.9, 34.9; n=22; Hoffman 2021). Here, we evaluated intensive SEA-BCMA dosing (Part B) and the addition of dexamethasone (DEX; Part C) to further assess the safety, tolerability, and clinical activity.
Methods Parts B and C enrolled patients (pts) who have received ≥3 prior lines of therapy for MM and are triple-class refractory. Responses are assessed per the 2016 International Myeloma Working Group criteria. These expansion cohorts are testing intensive dosing (Part B; weekly induction dosing of SEA-BCMA for 8 weeks is followed by Q2W maintenance dosing) and DEX combination therapy with either standard SEA-BCMA dosing (Part C1) or intensive SEA-BCMA dosing (Part C2; 800 mg [dose level -1] or 1600 mg [dose level 1]).
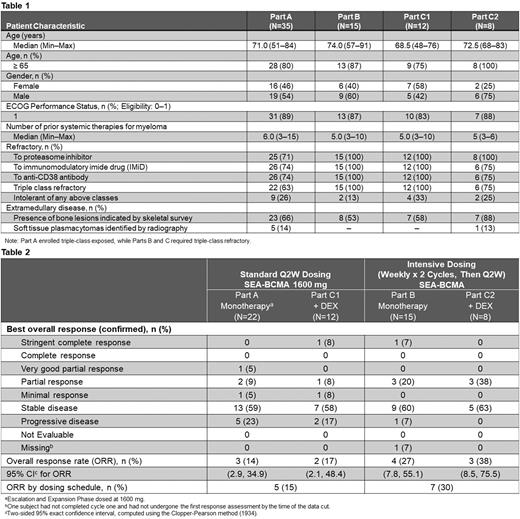
Results As of 16 May 2022, a total of 73 pts received SEA-BCMA. Patient baseline characteristics are summarized in Table 1.
The most common non-hematologic, treatment-emergent adverse events (TEAEs) in Parts B (n=15), C1 (n=12), and C2 (n=8) were fatigue (33%, 50%, and 25%), back pain (20%, 17%, and 25%), pneumonia (7%, 25%, and 25%), and cough (20%, 8%, and 25%). The most common hematologic TEAE was anemia (42% in Part C1). The most common ≥Grade 3 TEAEs were anemia (33% in Part C1) and pneumonia (7%, 25%, and 0% for Parts B, C1, and C2, respectively).
Both intensive SEA-BCMA dosing (Part B) and the addition of DEX (Part C1) showed increased ORRs relative to Q2W monotherapy (Part A; Table 2). In Part B (n=15), the ORR was 27% (95% CI: 7.8, 55.1); median duration of response (DOR) was 6.5 months (95% CI: 1.8, -); 3 pts remain on treatment, including 2 responders. In Part C1 (n=12), the ORR was 17% (95% CI: 2.1, 48.4); the median DOR was not yet reached; 1 responding pt remains on treatment.
For the combination of the intensive SEA-BCMA dosing schedule and the addition of DEX (Part C2), the ORR across both SEA-BCMA dose levels (800 mg [n=3] and 1600 mg [n=5]) was 38% (n=8; 95% CI: 8.5, 75.5); median DOR was not yet reached; 2 responding pts remain on treatment.
The combined ORRs of both intensive dosing cohorts (Parts B + C2) relative to both standard dosing (Q2W) cohorts (Parts A [1600 mg SEA-BCMA] + C1) were 30% versus 15%, respectively (Table 2).
Conclusions Updated results for SEA-BCMA continue to demonstrate a favorable safety profile and combination potential. Encouraging preliminary data suggest that both the intensive dosing schedule and combination with DEX may increase clinical activity over Q2W monotherapy. Part C2 and Part D (testing the combination of SEA-BCMA with pomalidomide and DEX in pts who have received ≥3 prior lines of therapy), are enrolling. Part C2 has been amended to allow pts with prior exposure to BCMA-directed therapy to enroll.
Disclosures
Hoffman:Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Seagen Inc.: Research Funding. Lipe:Janssen: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Sanofi: Consultancy; GSK: Consultancy; Seagen Inc.: Research Funding; Amgen: Research Funding; Harpoon: Research Funding. Melear:Janssen: Speakers Bureau; AstraZeneca: Speakers Bureau; TG Therapeutics: Speakers Bureau. Liedtke:Kura Oncology: Membership on an entity's Board of Directors or advisory committees; Natera: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Gilead: Research Funding; Caelum: Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Alnylam: Membership on an entity's Board of Directors or advisory committees; Allogene: Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Seagen Inc.: Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Schroeder:Incyte: Research Funding; Fortis: Research Funding; Cellect Inc: Research Funding; Genentech Inc: Research Funding; Seagen Inc.: Research Funding; Amgen: Research Funding; Celgene: Research Funding. Niesvizky:Takeda: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Yasenchak:Takeda: Research Funding; Seagen Inc.: Consultancy, Research Funding; Beigene: Speakers Bureau. Green:Seagen Inc.: Research Funding. Sauer:Seagen Inc.: Current Employment, Current equity holder in publicly-traded company. Wang:Seagen Inc.: Current Employment, Current equity holder in publicly-traded company. Ho:Seagen Inc.: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal